
Study: Covid-19 Vaccine Does Not Increase Risk Of Preterm Birth
Pregnant people who contract COVID-19 have an increased risk of disease severity and death, yet only 31 per cent of pregnant people in the United

Pregnant people who contract COVID-19 have an increased risk of disease severity and death, yet only 31 per cent of pregnant people in the United

Paris [France] (ANI/Sputnik): The French sanitary authority reported 104,611 new COVID-19 cases on Saturday, the highest daily toll seen in the country since the pandemic

Oscar-winning actor Matthew McConaughey doesn’t support a COVID-19 vaccine mandate for kids just yet. Nearly a week after the US Centers for Disease Control and
New Delhi [India] : India has administered over 107.92 crore COVID-19 vaccine doses so far under the nationwide vaccination drive, the Ministry for Health and

New Delhi [India] : Union Health Minister Mansukh Mandaviya on Wednesday said that the Centre has started ‘Har Ghar Dastak’ campaign to increase COVID-19 vaccination

Canberra [Australia] : Australian government on Monday gave recognition to Bharat Biotech’s Covaxin COVID-19 vaccine for the purpose of establishing a traveller’s vaccination status in

New Delhi [India] : Prime Minister Narendra Modi is set to interact with the seven Indian manufacturers of the COVID-19 vaccine on Saturday, days after

Taking a dig at Prime Minister Narendra Modi’s remark on VIP culture in vaccination campaign, All India Majlis-e-Ittehadul Muslimeen (AIMIM) chief Asaduddin Owaisi on Friday

New Delhi [India], October 22 (ANI): Delhi reported 22 new COVID-19 cases and zero deaths due to coronavirus on Thursday.A Delhi health department release said

New Delhi [India] : Union Commerce and Industry Minister Piyush Goyal on Thursday said that India crossing 100-crore COVID vaccinations is a big achievement and

The CoWin portal on Thursday mentioned that a total of 100 crore vaccine doses has been administered so far to the eligible population under the

New Delhi [India] : As India’s COVID-19 vaccination coverage surpassed the 100 crore mark on Thursday, Aam Aadmi Party chief and Delhi Chief Minister Arvind
New Delhi [India],3): The government has provided more than 103.5 crore COVID-19 vaccine doses to States and union territories (UTs), said the Union Health Ministry

As India achieved the milestone of 100 crore vaccination coverage, Prime Minister Narendra Modi said it was a historic day for the country and said
New Delhi [India] : More than 101.7 crore COVID-19 vaccine doses have been provided to states and union territories, out of which, over 10.42 crore
India will have a vaccine capacity of 28 crore doses this month, which includes 22 crore Serum Institute of India’s Covishield and 6 crore doses
Bharat Biotech on Friday said it has submitted all the data to the World Health Organisation (WHO) for Emergency Use Listing (EUL) of its COVID-19

Considering the burden on healthcare and the expense of buying three doses, Zydus Cadila is planning to seek approval for the two-dose regimen of its
The total active cases of COVID in India rose to 3,93,614, the ministry data showed.over 40,000 recoveries in 24 hours New Delhi [India], September 9
In a landmark achievement, India has administered 70,00,00,000 crore COVID-19 vaccine doses to date, informed Union Health Minster Mansukh Mandaviya on Tuesday. New Delhi [India],

New Delhi [India]: The national capital on Tuesday saw a slight surge in its daily count reporting 38 new COVID-19 cases and four fatalities in

With over 88.13 lakh COVID-19 vaccine doses administered in the last 24 hours, India has achieved the highest single-day vaccination mark on Monday. New Delhi
New Delhi [India], August 17 : Union Health Ministry on Tuesday informed that over 2.25 crore balance and unutilised COVID-19 vaccine doses are available with

Itanagar, Aug 13: Arunachal Pradesh Covid-19 caseload rose to 50,973 as 180 people have tested positive for the virus in the last 24 hours.According to

Vaccine production in the country has been increased from 2.5 lakh doses per day to around 40 lakh doses per day, said Dr Bharati Pravin

In order to ramp up COVID-19 vaccine supply in the country, Union Health Minister Mansukh Mandaviya on Friday met Serum Institute of India (SII) CEO
India needs time to review its legal provisions.US vaccines have landed in countries across the world, including Pakistan, Nepal, Bhutan and Bangladesh. The United States
Bengaluru : Karnataka Health and Medical Education Minister Dr K Sudhakar on Tuesday appealed to the people to be continue to be cautious warning that

Zydus Cadila seeks Emergency Use Authorisation for its DNA vaccine, ZyCoV-D, for 12 years and above in India. Zydus Cadila has applied for Emergency Use
Pregnant people who contract COVID-19 have an increased risk of disease severity and death, yet only 31 per cent of pregnant people in the United

Paris [France] (ANI/Sputnik): The French sanitary authority reported 104,611 new COVID-19 cases on Saturday, the highest daily toll seen in the country since the pandemic

Oscar-winning actor Matthew McConaughey doesn’t support a COVID-19 vaccine mandate for kids just yet. Nearly a week after the US Centers for Disease Control and
New Delhi [India] : India has administered over 107.92 crore COVID-19 vaccine doses so far under the nationwide vaccination drive, the Ministry for Health and

New Delhi [India] : Union Health Minister Mansukh Mandaviya on Wednesday said that the Centre has started ‘Har Ghar Dastak’ campaign to increase COVID-19 vaccination

Canberra [Australia] : Australian government on Monday gave recognition to Bharat Biotech’s Covaxin COVID-19 vaccine for the purpose of establishing a traveller’s vaccination status in

New Delhi [India] : Prime Minister Narendra Modi is set to interact with the seven Indian manufacturers of the COVID-19 vaccine on Saturday, days after

Taking a dig at Prime Minister Narendra Modi’s remark on VIP culture in vaccination campaign, All India Majlis-e-Ittehadul Muslimeen (AIMIM) chief Asaduddin Owaisi on Friday

New Delhi [India], October 22 (ANI): Delhi reported 22 new COVID-19 cases and zero deaths due to coronavirus on Thursday.A Delhi health department release said

New Delhi [India] : Union Commerce and Industry Minister Piyush Goyal on Thursday said that India crossing 100-crore COVID vaccinations is a big achievement and

The CoWin portal on Thursday mentioned that a total of 100 crore vaccine doses has been administered so far to the eligible population under the

New Delhi [India] : As India’s COVID-19 vaccination coverage surpassed the 100 crore mark on Thursday, Aam Aadmi Party chief and Delhi Chief Minister Arvind
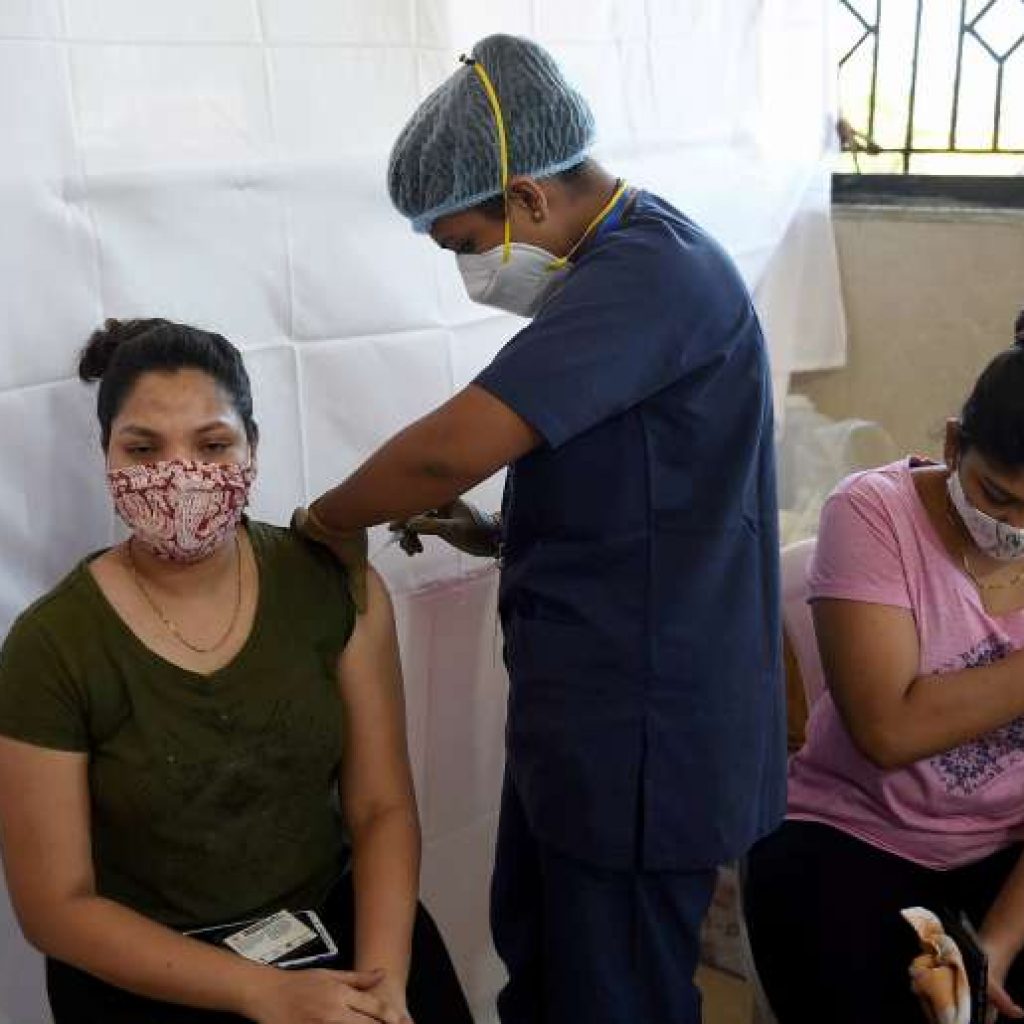
New Delhi [India],3): The government has provided more than 103.5 crore COVID-19 vaccine doses to States and union territories (UTs), said the Union Health Ministry

As India achieved the milestone of 100 crore vaccination coverage, Prime Minister Narendra Modi said it was a historic day for the country and said
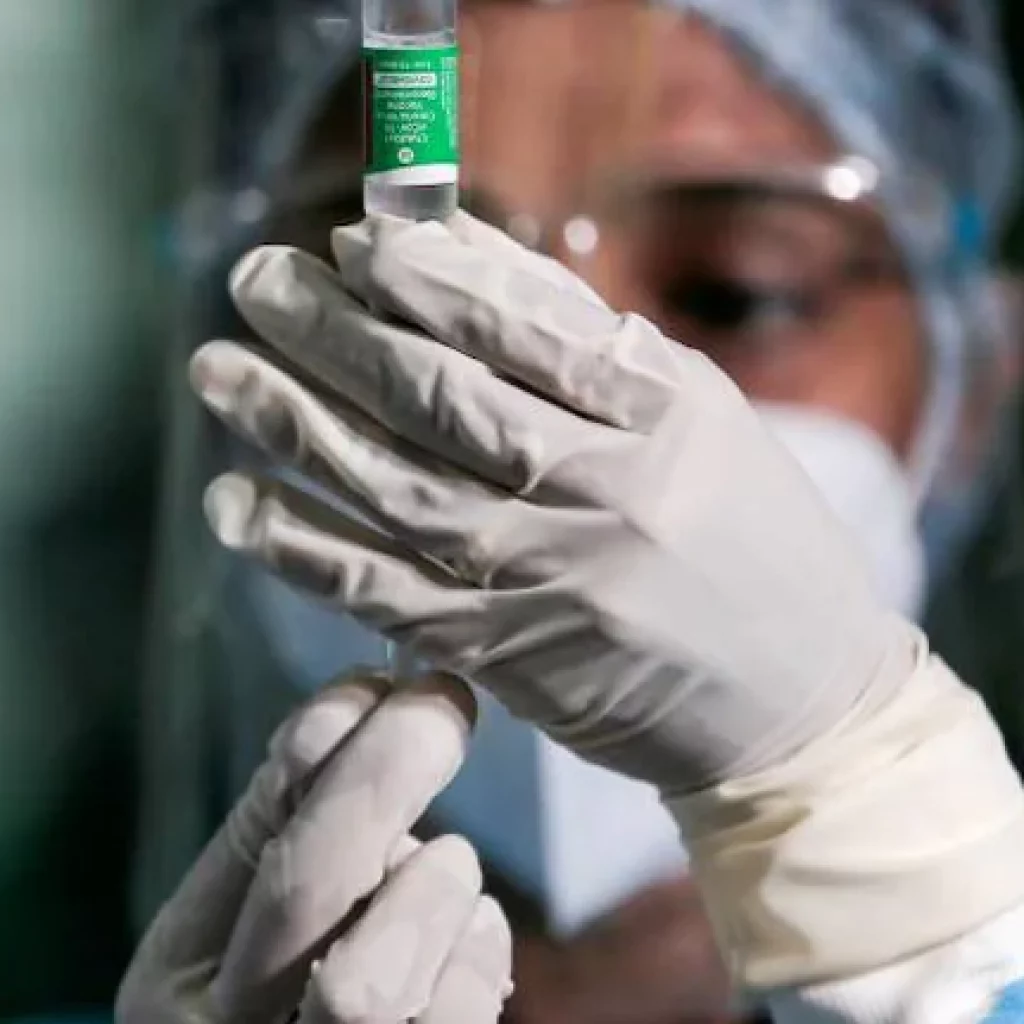
New Delhi [India] : More than 101.7 crore COVID-19 vaccine doses have been provided to states and union territories, out of which, over 10.42 crore
India will have a vaccine capacity of 28 crore doses this month, which includes 22 crore Serum Institute of India’s Covishield and 6 crore doses
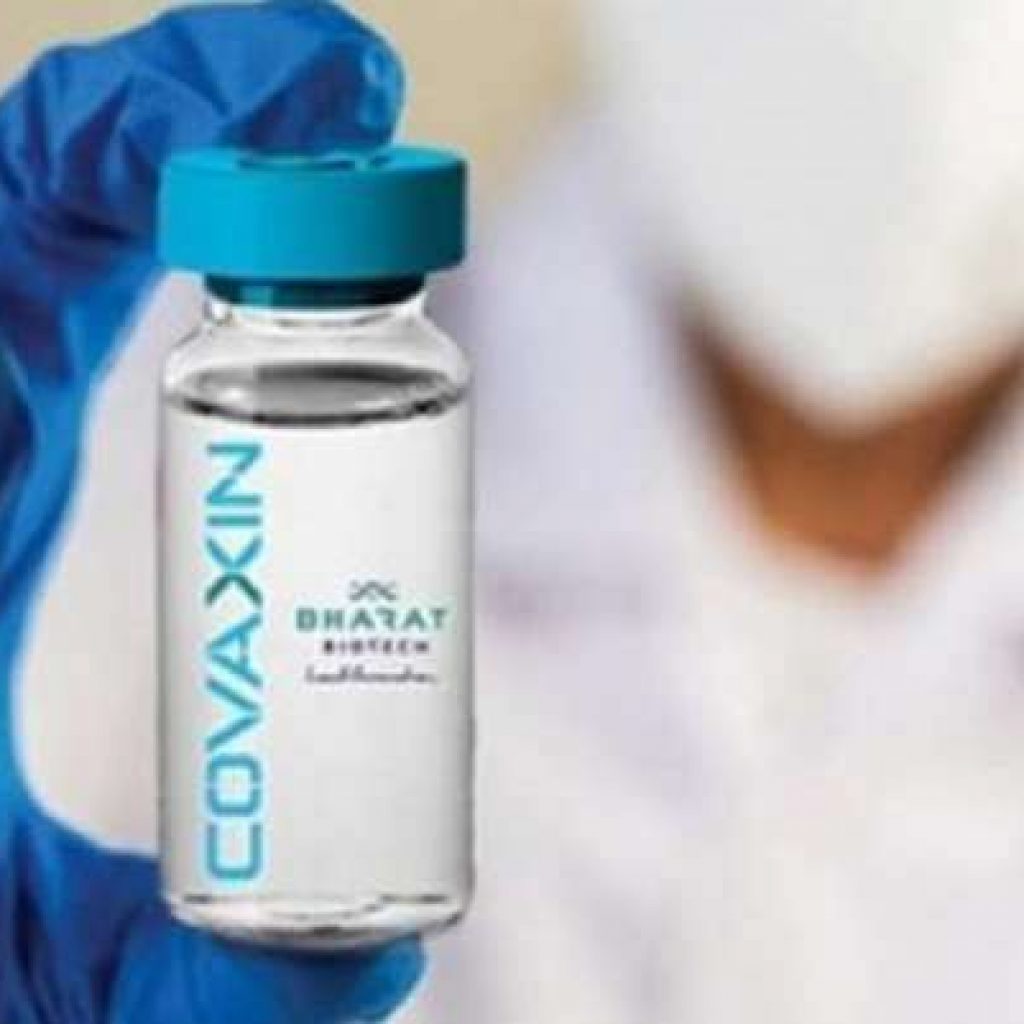
Bharat Biotech on Friday said it has submitted all the data to the World Health Organisation (WHO) for Emergency Use Listing (EUL) of its COVID-19

Considering the burden on healthcare and the expense of buying three doses, Zydus Cadila is planning to seek approval for the two-dose regimen of its
The total active cases of COVID in India rose to 3,93,614, the ministry data showed.over 40,000 recoveries in 24 hours New Delhi [India], September 9
In a landmark achievement, India has administered 70,00,00,000 crore COVID-19 vaccine doses to date, informed Union Health Minster Mansukh Mandaviya on Tuesday. New Delhi [India],

New Delhi [India]: The national capital on Tuesday saw a slight surge in its daily count reporting 38 new COVID-19 cases and four fatalities in

With over 88.13 lakh COVID-19 vaccine doses administered in the last 24 hours, India has achieved the highest single-day vaccination mark on Monday. New Delhi
New Delhi [India], August 17 : Union Health Ministry on Tuesday informed that over 2.25 crore balance and unutilised COVID-19 vaccine doses are available with

Itanagar, Aug 13: Arunachal Pradesh Covid-19 caseload rose to 50,973 as 180 people have tested positive for the virus in the last 24 hours.According to

Vaccine production in the country has been increased from 2.5 lakh doses per day to around 40 lakh doses per day, said Dr Bharati Pravin

In order to ramp up COVID-19 vaccine supply in the country, Union Health Minister Mansukh Mandaviya on Friday met Serum Institute of India (SII) CEO
India needs time to review its legal provisions.US vaccines have landed in countries across the world, including Pakistan, Nepal, Bhutan and Bangladesh. The United States
Bengaluru : Karnataka Health and Medical Education Minister Dr K Sudhakar on Tuesday appealed to the people to be continue to be cautious warning that

Zydus Cadila seeks Emergency Use Authorisation for its DNA vaccine, ZyCoV-D, for 12 years and above in India. Zydus Cadila has applied for Emergency Use